
cGMP HARPC for Food for Animals Compliance
InterlinkIQ ensures compliance with 21 CFR Part 507 cGMP HARPC for food for animals by automating documentation, risk assessments, and safety protocols. The software provides real-time monitoring and customizable reporting, ensuring adherence to regulatory standards. This enhances transparency, mitigates risks, and supports businesses in maintaining high-quality safety practices throughout the supply chain for animal food.

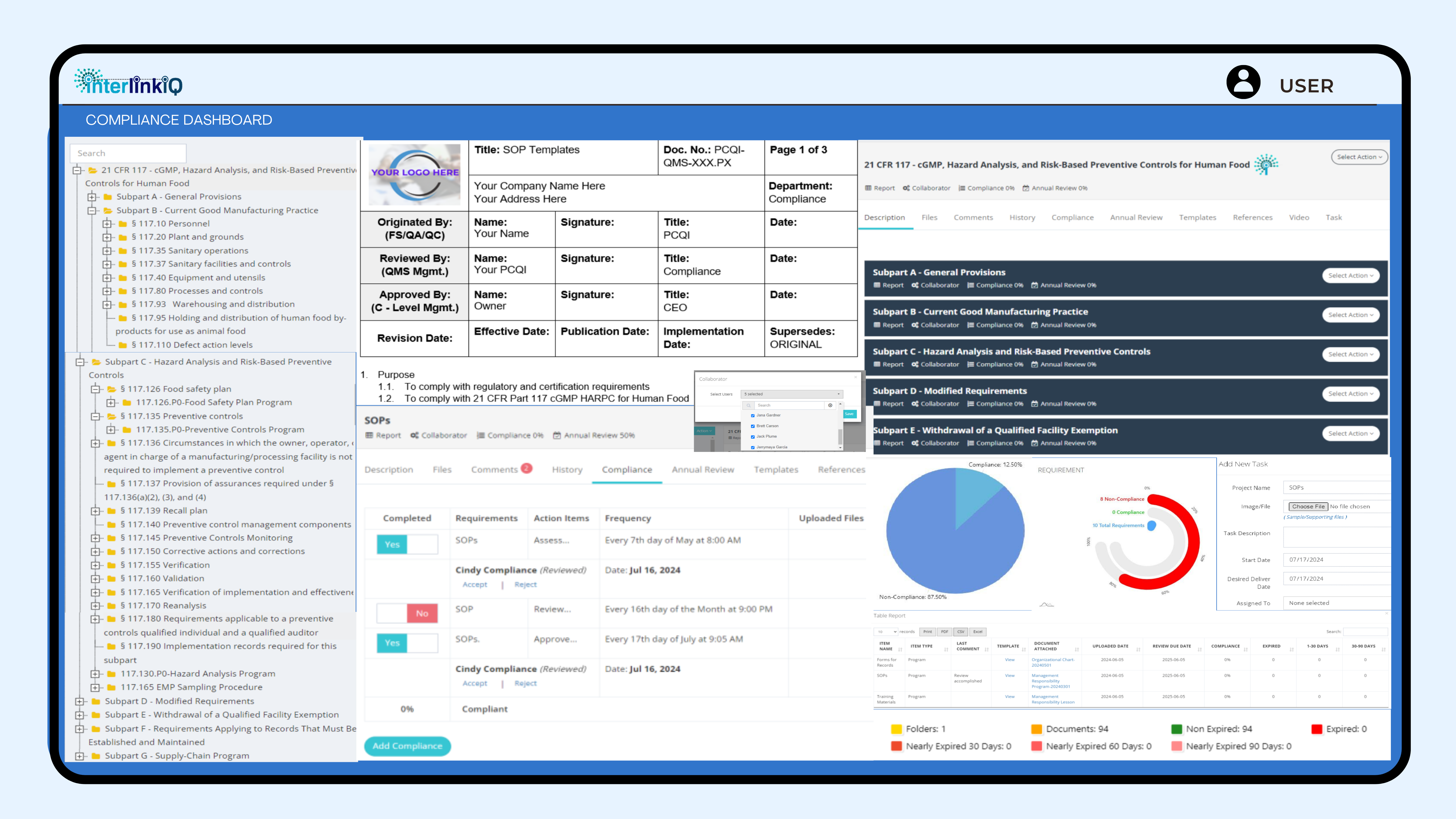
cGMP HARPC for Human Food
A comprehensive, user-friendly interface to track and manage all compliance requirements in accordance with 21 CFR Part 117 cGMP Hazard Analysis Risk-Based Preventive Controls for Human Food.
Access ready-to-use templates, real-time updates, customizable reports, and automated alerts. Includes essential documents for programs, policies, SOPs, record-keeping forms, and training materials. Stay ahead with proactive monitoring of due dates, compliance percentages, and nearly expired documents. Effortlessly manage and itemize compliance and annual review action items, ensuring you never miss a task. Simplify your compliance journey and achieve peace of mind with our all-in-one platform.


