
Dietary Supplements Compliance
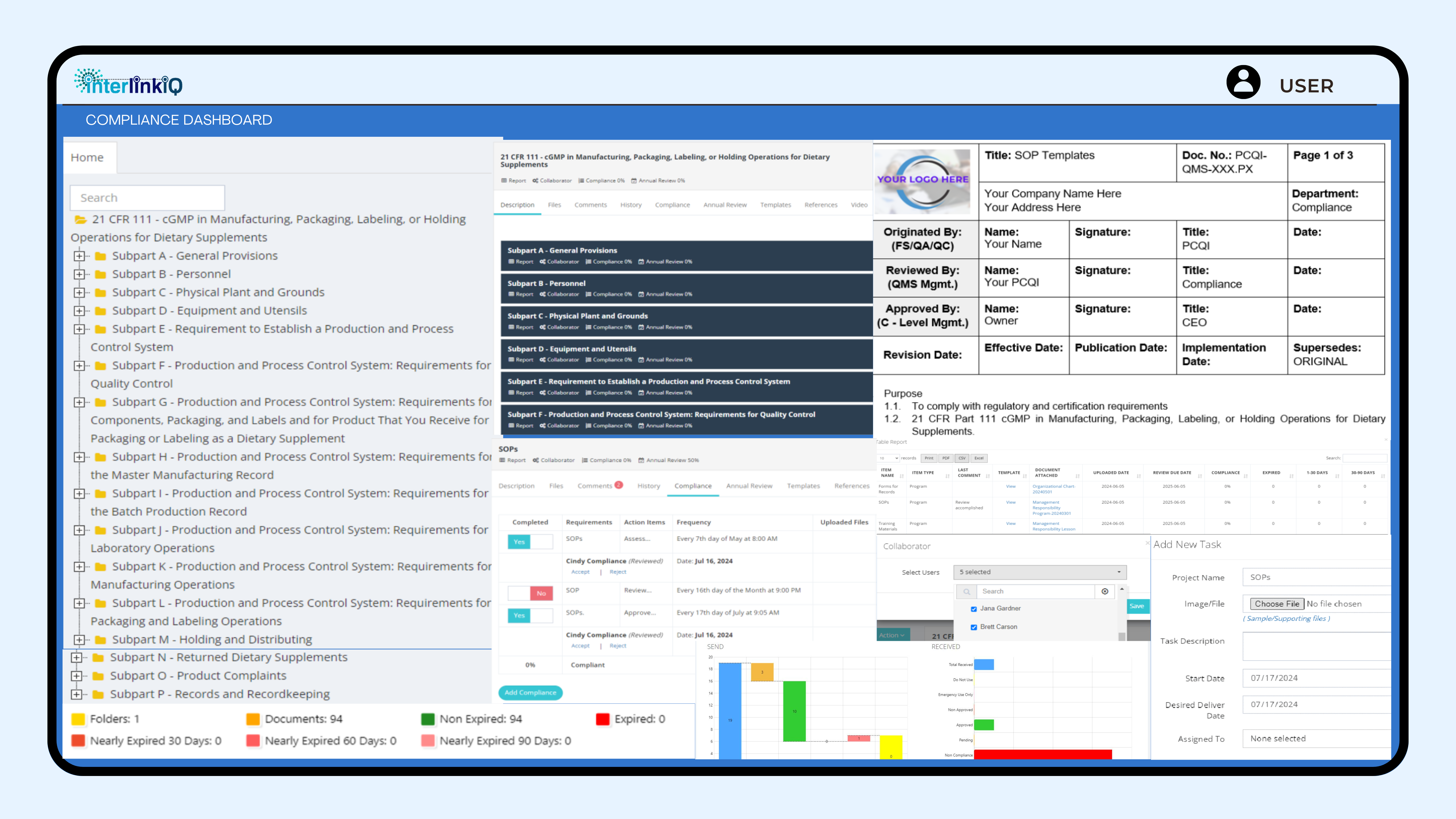
A comprehensive, user-friendly interface to track and manage all compliance requirements in accordance with 21 CFR Part 111 cGMP in Manufacturing, Packaging, Labeling, or Holding Operations for Dietary Supplements.
Offers templates, real-time updates, customizable reports, and automated alerts with documents (programs, policies, procedures (SOPs), forms for records, and training materials, monitors, review due dates, compliance percentages, nearly expired documents, along with itemized compliance and annual review action items, and task management.


